16 A1 16 2.4 cM Start 4,081,328 End 4,213,997 pattern Molecular function. Cellular component.

Mechanisms of transcriptional activation of cAMP. Review transcriptional activation by CREB proteins through. By the cAMP-responsive element-binding protein. Cyclic AMP-responsive element-binding protein 1. CAMP-responsive element-binding protein (CREB). Protein activation regulates transcriptional.
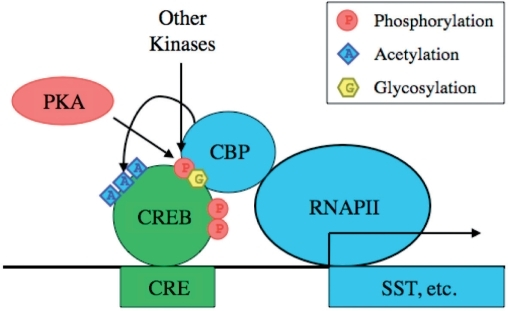
Biological process. Sources: / Orthologs Species Human Mouse RefSeq (mRNA) RefSeq (protein) n/a Location (UCSC) search CREB-binding protein, also known as CREBBP or CBP, is a that in humans is encoded by the CREBBP. The CREB protein carries out its function by activating transcription, where interaction with transcription factors is managed by one or more CREB domains: the interaction domain (RID), the ( and interaction domain), the / regions (TAZ1/CH1 and TAZ2/CH3) and the response binding domain (IBiD). The CREB protein domains, KIX, TAZ1 and TAZ2, each bind tightly to a sequence spanning both transactivation domains 9aaTADs of transcription factor p53.
Contents. Function This gene is ubiquitously expressed and is involved in the transcriptional of many different. First isolated as a nuclear protein that binds to -response element-binding protein , this gene is now known to play critical roles in embryonic development, growth control, and homeostasis by coupling chromatin remodeling to transcription factor recognition.
Philipp Hausseuffert
University Of Munster

The protein encoded by this gene has intrinsic activity and also acts as a scaffold to stabilize additional protein interactions with the transcription complex. This protein acetylates both histone and non-histone proteins. This protein shares regions of very high-sequence similarity with protein in its, cysteine-histidine-rich regions, and histone acetyltransferase domain. Recent results suggest that novel CBP-mediated post-translational N-glycosylation activity alters the conformation of CBP-interacting proteins, leading to regulation of gene expression, cell growth and differentiation, Posttranslational modification Homeodomain interacting protein kinase 2 phosphorylates several regions of CBP close to the N-terminal and close to the C-terminal region as well.
Out of the described phosphoacceptor sites, serines 2361, 2363, 2371, 2376, and 2381 are responsible for the HIPK2-induced mobility shift of the CBP C-terminal activation domain that is also visible in poly-acrylamide gel electrophoresis experiments. However, activation of CBP by HIPK2 is not mediated by this phosphorylation but rather by counteracting the repressive action of the cell cycle regulatory domain 1 (CRD1) of CBP, located between amino acids 977 and 1076. Clinical significance Mutations in this gene cause (RTS). Chromosomal translocations involving this gene have been associated with. Hypothalamic expression of this gene in mice correlates with mouse lifespan, and when CBP is inhibited in by, there is a proportional fold-change decrease in lifespan.
Small molecule inhibition A small molecule inhibitor (I-CBP112) binding to the bromodomain domain of CBP/p300 has been developed for leukaemia therapy. Interactions CREB-binding protein has been shown to with.